神经系统的炎症可以由多种因素导致,常见的有病毒或细菌感染、药物或有毒物质、自身免疫相关或特发性(原因不明的,如多发性硬化等)。神经系统的炎症不光导致感觉和运动异常,也可以表现为精神和行为障碍,如痴呆、精神分裂症等,因此,它与许多神经系统疾病密切相关,例如阿尔茨海默氏病(老年痴呆) 、帕金森氏病、多发性硬化等。
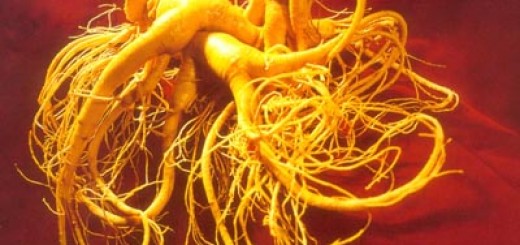
神经炎症与抑郁症和许多其他神经精神疾病密切相关。虽然针对炎症介质的神经系统药物表现出一定的疗效,但它面临着一些挑战,特别是药物输送和安全问题。最新的研究表明,从五加科植物中提取的人参达玛烷皂苷Rg1能够减轻神经系统炎症导致的精神行为障碍。
这个研究发现,外周的免疫调节剂可以有效地减轻神经发炎引起的异常行为,而这种免疫调节剂不需要渗透入脑(直接在外周血发挥作用)。在脑脂多糖(LPS)刺激的大鼠模型中,科学家也证实,一个行之有效的消炎制剂-人参达玛烷皂苷Rg1(达玛烷皂苷Rg1),无法穿透血脑屏障而进入大脑发挥作用。但有趣的是,只在外周血液和组织发挥免疫调节作用的达玛烷皂苷Rg1能有效地减轻受试大鼠的体重减轻,厌食症,抑郁症样行为,以及脑神经化学物质的异常。在外围神经免疫介质的生化分析表明,人参达玛烷皂苷Rg1可以减轻下丘脑 – 垂体 – 肾上腺轴的调节失控,并且选择性地减轻血液循环中白细胞介素-6水平的上升。与此相伴的是大脑内小胶质细胞活化的抑制,促炎介质和神经毒性物质的减轻。
总而言之,该研究表明,针对外周免疫系统的治疗可作为一种新方法,用于治疗神经炎症引起的神经精神障碍。例如,该研究为外周免疫调节剂达玛烷皂苷Rg1用于对抗精神障碍提供了依据。
原文发表于:Neuroscience. 2013 Oct 23;256C:210-222
Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats.
摘要:Neuroinflammatory disturbances have been closely associated with depression and many other neuropsychiatric diseases. Although targeting neuroinflammatory mediators with centrally acting drugs has shown certain promise, its translation is faced with several challenges especially drug delivery and safety concerns. Here, we report that neuroinflammation-induced behavioral abnormality could be effectively attenuated with immunomodulatory agents that need not to gain brain penetration. In a rat model with intracerebral lipopolysaccharide (LPS) challenge, we validated that ginsenoside Rg1 (Rg1), a well-established anti-inflammatory agent, was unable to produce a direct action in the brain. Interestingly, peripherally restricted Rg1 could effectively attenuate the weight loss, anorexic- and depressive-like behavior as well as neurochemical disturbances associated with central LPS challenge. Biochemical assay of neuroimmune mediators in the periphery revealed that Rg1 could mitigate the deregulation of the hypothalamic-pituitary-adrenal axis and selectively blunt the increase in circulating interleukin-6 levels. Furthermore, these peripheral regulatory effects were accompanied by dampened microglial activation, mitigated expression of pro-inflammatory mediators and neurotoxic species in the central compartment. Taken together, our work suggested that targeting the peripheral immune system may serve as a novel therapeutic approach to neuroinflammation-induced neuropsychiatric disorders. Moreover, our findings provided the rationale for employing peripherally active agents like Rg1 to combat mental disturbances.