Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. The CNS is typically an immunologically privileged site because peripheral immune cells are blocked by the blood brain barrier (BBB). However, circulating peripheral immune cells and immunomediators (e.g. interleukins, etc.) may surpass a compromised BBB and encounter neurons and glial cells, perpetuating the immune response.
Neuroinflammation is widely involved in pathogenesis of a number of brain and spinal cord diseases, including Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Because neuroinflammation has been associated with a variety of neurodegenerative diseases, there is increasing interest to determine whether reducing inflammation will reverse neurodegeneration. Inhibiting inflammatory cytokines, such as IL-1β, decreases neuronal loss seen in neurodegenerative diseases.
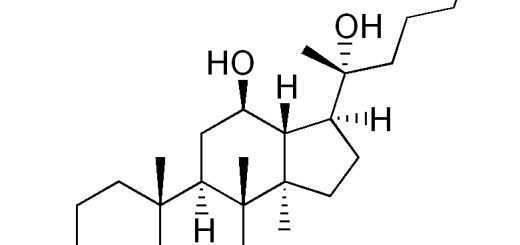
Scientists from China Pharmaceutical University demonstrated that ginsenoside Rg1 could modulate the peripheral immne response to influence indirectly the inflammation in central nervous system. The finding was published on “Neuroscience, 2014;256:210-222”, a well-reputated journal in neurology field.
Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats.
Abstract:
Neuroinflammatory disturbances have been closely associated with depression and many other neuropsychiatric diseases. Although targeting neuroinflammatory mediators with centrally acting drugs has shown certain promise, its translation is faced with several challenges especially drug delivery and safety concerns.
Here, we report that neuroinflammation-induced behavioral abnormality could be effectively attenuated with immunomodulatory agents that need not to gain brain penetration.
In a rat model with intracerebral lipopolysaccharide (LPS) challenge, we validated that ginsenoside Rg1 (Rg1), a well-established anti-inflammatory agent, was unable to produce a direct action in the brain. Interestingly, peripherally restricted Rg1 could effectively attenuate the weight loss, anorexic- and depressive-like behavior as well as neurochemical disturbances associated with central LPS challenge.
Biochemical assay of neuroimmune mediators in the periphery revealed that Rg1 could mitigate the deregulation of the hypothalamic-pituitary-adrenal axis and selectively blunt the increase in circulating interleukin-6 levels.
Furthermore, these peripheral regulatory effects were accompanied by dampened microglial activation, mitigated expression of pro-inflammatory mediators and neurotoxic species in the central compartment.
Taken together, our work suggested that targeting the peripheral immune system may serve as a novel therapeutic approach to neuroinflammation-induced neuropsychiatric disorders. Moreover, our findings provided the rationale for employing peripherally active agents like Rg1 to combat mental disturbances.